

The Optics Laboratory
Group of
Hans Hallen, North Carolina State University Physics Department

Raman Spectroscopy
- Inelastic collision
- Light loses (or gains) energy due to vibration energy level change in sample.
- Raman-active if polarizability changes as the molecules move during the vibration.
- The chance of photon interaction is small (~1 in a million), so one usually counts the Raman scattered photons instead of measuring the intensity on a DC meter.

Green light comes in from the left. Some of the energy of one photon is absorbed by the molecule, changing its color to yellow. (That is an exaggeration, for most materials it would still look almost the same shade of green.) The purple arrow shows the change in the molecule, as the vibration amplitude (red arrow) gets larger.
IR Spectroscopy
- Absorption of IR light
- Beer-Lambert Law (the light intensity decreases exponentially as it passes through the material).
- IR-active if polarization changes during the vibration
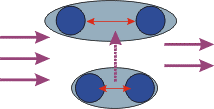
Purple light comes in from the left. All the energy of one photon is absorbed by the molecule, annihilating the photon. The purple arrow shows the change in the molecule, as the vibration amplitude (red arrow) gets larger.
Apparatus for Raman
- A large (~1m) grating spectrometer is used to measure the small color shanges. Great care must be taken to extract the signal in the presence of the large
non-Raman-scattered light by
- using a 2 or 3-stage spectrometer, in which the output stage of one spectrometer is fed into another so it acts as a better light filter.
- using a holographic notch filter to filter out the elastically scattered light before sending the light into a 1-stage spectrometer.
- Detection for the low light levels. This usually involves photon counting.
- A special grade of photomultiplier tube can be used to count photons. The spectrometer is set to pass just one narrow range of light at a time, and the photon energy (usually measured in units of inverse wavelength = wavenumers) is scanned.
- A CCD camera can be used. This imaging detector can measure the number of photons in a range of photon energies all at once, so is faster.
- A parameter of importance in the detector is the dark-count. This is the number of 'false' counts in the detector due to thermal excitation. Either 2 or 3-stage Peltier coolers or liquid nitrogen stages are used.
- Currently operating at -45°C
- Approximately 0.5 count/sec noise background
- A laser operates as the light source, to increase the signal level due to the inherent brightness of the laser light.

This schematic drawing of one of our Raman set-ups illustrates a typical system. Laser light is coupled into an optical fiber, which carries the light to the sample. Scattered light is collected and collimated by a lens, and passes through the notch filter before being focussed onto the entrance of the spectrometer. The light is dispersed by the grating, and is detected by the cooled CCD camera.
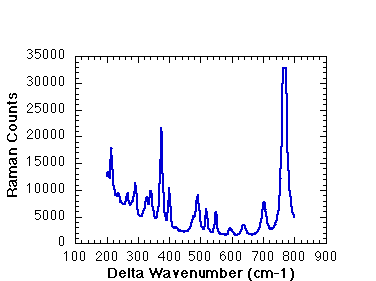
This Raman spectrum, of a potassium titanyl phosphate, has many vibration modes that are Raman active. Each peak corresponds to a different vibration in the material. The energy of the vibration increases to the right. Note the background on the left side of the plot. This is due to elastic (mainly Rayleigh ) scattering from defects in the crystal, and is known as the Rayleigh tail.

More info is in the papers.

NC State University | Physics | Optics Home
Last updated on September 29, 2000
Copyright ©1999, Hallen Laboratory, NCSU, Raleigh, NC. www.physics.ncsu.edu/optics
Comments or questions? Hans_Hallen@ncsu.edu
![]()
![]()
![]()
![]()
![]()
![]()




![]()
![]()