J Anat. 2013 Jan; 222(1): 147–160. Evolution of the mammalian middle ear and jaw: adaptations and novel structures
1Division of Developmental Neurobiology, MRC National Institute for Medical Research, London, UK
2Department of Craniofacial Development and Stem Cell Biology, King’s College London, Guy’s Hospital, London, UK
Abigail S. Tucker, Department of Craniofacial Development and Stem Cell Biology, King’s College London, Floor 27 Guy’s Tower, Guy’s Hospital, London Bridge, London SE1 9RT, UK. E:
ku.ca.lck@rekcut.liagibaCopyright © 2012 The Authors. Journal of Anatomy © 2012 Anatomical Society
This article has been
cited by other articles in PMC.
Abstract
Having three ossicles in the middle ear is one of the defining features of mammals. All reptiles and birds have only one middle ear ossicle, the stapes or columella. How these two additional ossicles came to reside and function in the middle ear of mammals has been studied for the last 200 years and represents one of the classic example of how structures can change during evolution to function in new and novel ways. From fossil data, comparative anatomy and developmental biology it is now clear that the two new bones in the mammalian middle ear, the malleus and incus, are homologous to the quadrate and articular, which form the articulation for the upper and lower jaws in non-mammalian jawed vertebrates. The incorporation of the primary jaw joint into the mammalian middle ear was only possible due to the evolution of a new way to articulate the upper and lower jaws, with the formation of the dentary-squamosal joint, or TMJ in humans. The evolution of the three-ossicle ear in mammals is thus intricately connected with the evolution of a novel jaw joint, the two structures evolving together to create the distinctive mammalian skull.
Keywords: evolution mammals, jaw joint, middle ear
Introduction
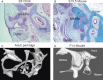
The middle ear ossicles in mammals sit in an air-filled cavity and bridge the gap between the external and inner ear. Vibrations in the tympanic membrane (ear drum) are picked up by the manubrium of the malleus and transferred to the incus and stapes, which then conducts the vibrations to the inner ear via the oval window (). Defects in this process lead to conductive hearing loss. In birds and reptiles, a single ossicle spans the air-filled middle ear cavity, transferring vibrations from the external to inner ear. In birds this ossicle is known as the columella auris, while in reptiles it is known as the stapes ().
Middle ear ossicles in mammals and birds. (A) Frontal section through the developing middle ear of a chick showing the columella (c) spanning the gap between the external and internal ear at E (embryonic day) 6. (B) Sagittal section through the developing ...
The mammalian middle ear ossicles are housed in the auditory bulla, a bony capsule that protects the ear and defines the cavity. The bulla is made of a number of bones, including the tympanic ring that supports the tympanic membrane. The tympanic ring is a membranous bone that forms in close association with Meckel’s cartilage and the malleus. The smaller gonial bone lies in between the tympanic ring and the malleus and has an important role as an investing bone for the malleus. The malleus is therefore a compound bone with a dual origin from endochondrial ossification and from invasion of bone from the gonial.
As reptiles and birds only have one ossicle, homologous to the mammalian stapes, the homologous skeletal elements to the malleus, incus, tympanic ring and gonial have been a subject of much discussion. Where did these extra ossicles and bones come from?
In 1837, Reichert proposed that the malleus and incus were homologous to the articular and quadrate of the non-mammalian jaw joint based on anatomical comparisons (Reichert, 1937). In 1912, Gaupp extended Reichert’s theory and described the development of a primary jaw joint between the malleus and incus and a secondary jaw joint between two membraneous bones, the squamosal and dentary, that was unique to mammals (Gaupp, 1912). Other theories have been proposed and rejected, but Reichert’s theory has subsequently been supported by a wealth of information from the fossil record, from developmental biology and molecular biology and from the study of marsupials. Together, research in these areas has produced a united theory of the steps and possible mechanisms involved in creating the unique mammalian ear and jaw.
Evidence from developmental biology
Meckel’s cartilage appears as two rods of hyaline cartilage that traverse the lateral aspect of the mandible. In non-mammalian vertebrates the most proximal part of Meckel’s forms the articular and quadrate (or palatoquadrate). These are endochondrial bones between which the jaw joint forms, articulating the upper and lower jaw. From fate-mapping studies between quail and chick embryos the articular part of Meckel’s and the quadrate have been shown to be derived from the first pharyngeal arch (Couly et al. 1993; Kontges & Lumsden, 1996). The retroarticular process that develops proximally to the articular, and the body of the columella are derived from the second pharyngeal arch. The quadrate and articular are initially derived from a single cartilaginous condensation that subdivides to form the two skeletal elements separated by the jaw joint (Wilson & Tucker, 2004). If the malleus and incus are homologous to the articular and quadrate, we would expect a similar pattern of embryonic development.
Just as observed with the articular and quadrate, the malleus and incus are endochondrial bones initially united as a single cartilaginous condensation, which subdivides into the two ossicles (Amin & Tucker, 2006). In contrast, the stapes is derived from a separate condensation that grows towards the incus to form a joint. Like the articular and quadrate, the malleus and incus form from the posterior part of Meckel’s cartilage and the malleus, like the articular, remains attached to Meckel’s during much of embryonic development, forming a direct connection between mandible and middle ear ().
Comparison of membranous bone ossification in the middle ear and jaw joint. Alcian Blue and Alizarin Red-stained skeletal preparations. (A–C) Development of the cartilages and bones of the murine middle ear, side view. (D–F).Development ...
The incus retains a thin connective tissue link, visible by histology, to the ala temporalis, which is thought to be homologous to the ascending process of the palatoquadrate (Presley & Steel, 1978). In the mouse the cartilage connection between the jaw and ear only breaks down postnatally, starting at around P2, with the transformation of Meckel’s cartilage next to the malleus into the sphenomandibular ligament. This breakdown of Meckel’s cartilage is an important step in mammals as it allows functional separation of the ear from the jaw, and it will be discussed in more detail later. In non-mammalian species, in contrast, Meckel’s cartilage remains continuous, forming a core support for the membraneous bones that ossify along its length, from symphysis to articulation point.
Fate-mapping studies using a Hoxb1-cre reporter mouse have shown that the processus brevis at the bottom of the malleus and the stapes are second arch-derived (O’Gorman, 2005). The fact that the mammalian stapes and the bird columella are both second arch-derived again strengthens the homology between these two ossicles. This result also indicates that the processus brevis on the end of the malleus is homologous to the second arch-derived retroarticular process on the end of the articular. This is an intriguing finding as from classical comparative anatomy and fossil data it had been suggested that the retroarticular process was homologous to the manubrium of the malleus (Kermack & Musset, 1983; Allin & Hopson, 1992). The fate-mapping experiments, however, argue strongly against this and suggest that the manubrium is a novel mammalian structure without a homologue in birds and reptiles.
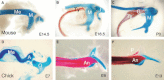
Reichert’s theory has also been backed up by analysis of gene expression. For example, Bapx1 is a jaw joint marker, expressed in the developing articular-quadrate joint in birds, fish and reptiles (Miller et al. 2003; Wilson & Tucker, 2004) (). In mammals, however, Bapx1 is found associated with the malleal-incudo joint in the developing middle ear (Tucker et al. 2004) (). The malleus/articular and incus/quadrate therefore share a common developmental history, tissue origin, and gene expression pattern (schematised in ).
Conservation of Bapx1 expression between the quadrate and articular and malleus and incus in a bird, reptile and mammal. Sagittal sections through the developing jaw joint in (A,B) Chick and (C,D) Python, and through the middle ear in the mouse (E,F). ...
Schematic of a chick and mouse head during late development. Homologous structures are shown in the same colour.
Developmental biology can also help identify the homologous elements for the associated membranous bones of the ear. The tympanic ring and gonial, for example have been suggested to be homologous to the angular bone and prearticular, respectively. Again, by following the position and relative timing of these bones as they develop, clear homologies can be identified (). Bapx1 is also expressed around these membraneous bones in both chick and mouse (Tucker et al. 2004).
The malleus and incus ossify relatively early in development, fixing their size in contrast to the growing cranium and mandible. In this way the ossicles remain small while the head grows. This negative allometry can be followed clearly in marsupial neonates, as the malleus and incus change from a jaw-supporting to hearing role (as outlined in Luo, 2011). It has been suggested that this early ossification had a central role in changing the relative size of the malleus and incus with respect to the head but might have also led to the posterior displacement of these ossicles necessary for isolation of the ear from the jaw.
Evidence from mouse mutants
A number of mouse mutants have been created which result in re-shaping of the middle ear region, leading to the development of dysmorphic ossicles, or even transformations of ossicle type due to a change in patterning of the mandible and maxilla. In Dlx5/6 double knockout mice the mandible is transformed into the identity of the maxilla, leading to the formation of rugae (palatal ridges) and vibrissae development on the lower jaw (Depew et al. 2002). The endothelin pathway acts upstream of the Dlx genes and a similar transformation of jaw identity is observed after knockout of the endothelin receptor (Ednra; Clouthier et al. 1998; Ozeki et al. 2004; Ruest et al. 2004). In both Dlx and endothelin receptor mutants the tympanic ring and gonial are lost and the malleus is dysmorphic, possibly showing a transformation to an incus morphology (Ozeki et al. 2004; Depew et al. 2005). An equally dramatic reverse transformation of maxilla to mandible is observed when the endothelin receptor is made constitutively active (Sato et al. 2008). In this knockin mouse, Meckel’s cartilage from the two mandibles meet to form a middle ear with two tympanic rings, and two gonials and a possible duplication of the malleus (Sato et al. 2008). These mutants strengthen the idea that the joint between the incus and malleus is the pivotal point between the upper and lower jaws in the mouse; thus although the articulation site has moved, the genetic regulation of upper and lower fate is still controlled around the primary jaw articulation. In Hoxa2 mutants, the second arch is transformed into a proximal first arch, and the second arch-derived structures such as the stapes and Reichert’s cartilage are missing. In their place, an ectopic malleus, incus and tympanic ring form (Gendron-Maguire et al. 1993; Rijli et al. 1993). In addition, an ectopic cartilage was found connected to the incus that was suggested to be a palatoquadrate, the homolog of the incus in primitive vertebrates (Rijli et al. 1993). A similar ectopic palatoquadrate has been observed in Dlx2 mutants (Qiu et al. 1995). These mutants were therefore proposed to show a skull pattern more reminiscent of the basic synapsid skull of a pre-mammalian ancestor. It has been argued, however, that these ectopic cartilages do not represent true atavisms and are secondary consequences of disruptions in cell specification, migration and/or differentiation (Smith & Schneider, 1998). Such changes might cause the chondrification of the connective tissue thread that links the incus to the ala temporalis. The identity and significance of such ectopic cartilages are therefore unclear. In the Hoxa2 knockout, the ectopic middle ear elements develop as mirror image versions of the normal first arch-derived middle ear skeletal structures and fuse with them at the point where the first and second arch crest normally meet. For example, the ectopic malleus fuses with the normal malleus at the position of the second arch-derived processus brevis, which is lost (O’Gorman, 2005). A distinct origin for the processus brevis, relative to the rest of the malleus, is highlighted in the Msx1 mutant, where the first arch-derived body of the malleus and manubrium are normal but the second arch processus brevis is lost (Satokata & Maas, 1994). Other mutants show loss of specific regions of the middle ear, shedding light on how identity of the ossicles might be regulated. For example, the incus is specifically lost in the Emx2 mutant, leaving the malleus and incus relatively unaffected (Rhodes et al. 2003). Emx2 is expressed within and around the developing incus (Amin & Tucker, 2006). Given the distinct role for Emx2 in the incus, it would be predicted that in a non-mammalian gnathostome Emx2 would be expressed in the quadrate/palatoquadrate. Interestingly Emx2 has been shown to be expressed in distinct regions in the developing pharyngeal arches in dogfish and Xenopus embryos (Derobert et al. 2002; Galli et al. 2003). Whether the expression domain corresponds to the palatoquadrate during later development, however, is unclear, with expression in the dogfish suggested to be in the mesoderm rather than the neural crest (Derobert et al. 2002).
Evidence from fossils
Although somewhat sparse, the fossil evidence for the evolutionary transition of the articular-quadrate jaw articulation to the malleal-incodal middle ear joint is amongst the most complete of any anatomical transition (as reviewed extensively in Luo, 2011). The emergence of mammal-like reptiles (mamalliforms) occurred in the cynodont synapsid lineage, a group that gave rise to modern mammals as well as extinct ancestors and closely related species (Luo, 2011). In fact all extant mammals are descendants of just three Mesozoic lineages (placental, monotreme and marsupial) from a compliment of more than 20 extinct mammalian lineages (Kielan-Jaworowska et al. 2004; Benton, 2005).
In such transitional fossils it is clear that the first step in the process of formation of a mammalian-like ear and jaw was the development of a double jaw joint, that is, two side by side joints, one between the articular and the quadrate and the other between the dentary and the squamosal. An extended upward dentary, which perhaps provided a step towards formation of such a second joint, has been observed in the non-mammalian synapsids, Scymnognathus and Ictidopsis (Hall, 2005; Kemp, 2005). In tritheledontids and brasilodontids, two advanced mammal-like synapsid groups, the dentary has a lateral ridge that contacts the ventral side of the squamosal forming a functional hinge but does not have a well developed articulation (Luo, 2011). A clear double jaw joint is evident in the prototypical basal mammaliform Morganucodon from the Jurassic. In synapsids such as Morganucodon the articular-quadrate joint is attached to both an ossified Meckel’s cartilage and dentary bone (Kermack et al. 1981). Morganucodon has conical cusps on its teeth, with numerous accessory cusps, and importantly the teeth are elongated along the line of the jaw with multiple roots. Unlike the teeth of reptiles, these teeth would have functioned as a shearing mechanism. The formation of a double joint may have had the advantage of providing resistance against the forces produced by this searing dentition, which would have introduced a twisting motion to the jaw. Such a twisting would result in a tendency to dislocate the jaw articulation, prevented by the presence of the double articulation (Kermack, 1972; Crompton & Hylander, 1986; Kemp, 2005). The advent of a double jaw joint was therefore directly linked to a change in tooth shape and change in mode of mastication. A similar double jaw joint has been described in Kuehneotherium, which has been suggested to be the ancestor of placental and marsupial mammals, while Morganocodon has been suggested to be related more closely to monotremes. In both cases, however, the mandible is a compound structure and the Q-A joint pronounced.
The postdentary middle ear bones (angular, prearticular) in early mammaliforms are housed in a trough in the dentary bone. In many fossils the small middle ear ossicles and associated bones are lost but the trough in the dentary is taken as evidence of their existence and attachment to the dentary. Such a postdentary trough is observed in Morganucodon. In a similar manner, the presence of a Meckel’s groove on the dentary has been used as evidence of a persistent Meckel’s cartilage.
The next step after evolution of a double articulation appears to have been detachment of the postdentary bones from the dentary, as evidenced by lack of a postdentary trough. These postdentary bones would have still been connected to the jaw via an ossified Meckel’s cartilage. It is important to note that lack of a postdentary trough does not automatically mean that the middle ear bones were detached from the jaw (Ji et al. 2009). Recently, an unambiguous example of a transitionary form was described in the form of Liaoconodon, an eutriconodont mammal from the early cretaceous. Here, the malleus and ectotympanic are preserved and detached from the dentary while maintaining their connection to an ossified Meckel’s cartilage. An ossified Meckel’s is also found in several gobiconodontids, the Spalacotheroid Maotherium and the eutriconodont Yanoconodon (Wang et al. 2001; Luo et al. 2007; Ji et al. 2009). Detachment therefore appears to have occurred independently in a number of different lineages during evolution and can be regarded as a frequent homoplasy (Luo, 2011). It is thought that Meckel’s was ossified to provide a support for the middle ear ossicles, before their connection to the cranium (Meng et al. 2011). The displacement of the malleus and incus from the lower jaw indicates their increasing specialisation as auditory ossicles, though not unaffected by chewing, a consequence of the fusion to an ossified Meckel’s cartilage.
The final step in formation of the definitive mammalian middle ear appears to have been a breakdown of Meckel’s cartilage, so that the ear is no longer physically connected to the jaw. This is linked with support for the ear by connection to the cranium, with the formation of the auditory bulla. Such a situation is observed in Hadrocodium, which does not have a postdentary trough or Meckel’s groove. The only jaw articulation is between the dentary and squamosal and the ear would appear to be completely free of the jaw. Interestingly, Hadrocodium has a large brain case, indicating that indeed an increase in brain size might have influenced the separation of the middle ear bones from the jaw (Luo et al. 2001), agreeing with the proposal of Rowe (1996). In Repenomamus, however, the brain case is small, disagreeing with the theory that detachment of the postdentary bones from the jaw was driven by expansion of the brain (Wang et al. 2001).
In birds and reptiles the tympanic membrane (ear drum) is supported by the quadrate. As this bone reduced in size and became incorporated into the middle ear, a new support was necessary for the tympanic membrane. From the fossil record it appears that the angular bone took over this role, transforming from a lower jaw support to the tympanic ring. In cynodonts the angular is plate-like, with a large surface area for receiving sound. Whether the mammalian and non-mammalian tympanic membranes are homologous is still an area of dispute, with discrepancies involving the position of the muscles in relation to the Eustachian tube indicating that the two ear drums may not be homologous (reviewed in (Takechi & Kuratani, 2010)).
Evidence from marsupials
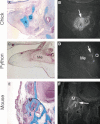
Although the fossil record provides clues to the transition it is often incomplete and relies on a few isolated specimens. The ideal solution would be to be able to follow the transition from primary to novel articulation in a living animal. This is indeed possible in marsupials (Maier, 1987). In marsupials the neonate must be able to suckle at an early developmental stage, prior to the formation of the bones that will make up the normal mammalian jaw joint. Marsupials, therefore utilise the joint between the incus and malleus as their primary jaw joint for the first few weeks after birth (Muller, 1968) (). A clear synovial joint between the malleus and incus has been reported at postnatal day (P) 3 in the opossum Monodelphis (Filan, 1991). The degree that this middle ear joint is actually functional in the newborn is debatable, and much of the suckling action is thought to be achieved by the flexibility of Meckel’s cartilage (Filan, 1991). The dentary, squamosal and condylar cartilage then start to form and a double joint is visible, one between the incus and malleus and one between the squamosal and dentary. The connection between the malleus and Meckel’s cartilage is lost by P20, with the squamosal and dentary taking over the role of jaw joint. At this stage the malleus and incus are still relatively large and attached to the brain case but over the following weeks they become incorporated into the middle ear (Filan, 1991; Clark & Smith, 1993; Smith, 2006). During this transition the muscles change, exemplified by the changing shape and size of the tensor tympani, which inserts on the malleus. In the opossum neonate at P0, the cells of the tensor tympani are found in a continuous mass with the internal pterygoideus (one of the jaw-closing muscles which inserts on the angular process of the dentary). Both muscles are of a similar size at this time point. As the malleus and incus shift from their role as support for the dentary to hearing, the tensor tympani changes from a large mass of fibres to a small muscle inserting on the malleus, whereas the pterygoid greatly increases in size (Smith, 1994). This change in size represents a change in function from a major support of the jaw to an ear-drum tensing role within the middle ear. Marsupials therefore provide a great resource for following the transition from primary to novel jaw articulation. The marsupial use of the primary jaw joint has been cited as an example of ‘von Baer’s recapitulation’, based on the fact that the marsupial neonate resembles that of the embryonic condition of mammalian ancestors (Maier, 1990; Sanchez-Villagra et al. 2002).
Jaw joint comparison in the mouse, chick and the marsupial Monodelphis. Skeletal preps. Red – bone stained by Alizarin Red. Blue – cartilage stained by Alcian Blue. (A) Mouse postnatal day (P) 0. (B) Chick embryonic day (E) 13. (C) Monodelphis ...
The secondary jaw joint
Utilisation of the articular, quadrate, angular and prearticular in the mammalian ear would not be possible without the evolution of a new jaw articulation between the squamosal and dentary (). The benefits of the squamosal-dentary joint, providing a robust, load-bearing articulation point, have been argued to be the driving force in the evolution of the mammalian ear (Crompton, 1963; Crompton & Hylander, 1986). In this scenario, supported by the fossil record, the new jaw joint would have come first, freeing up the primary jaw joint to play a role in the middle ear as a secondary consequence. For the successful creation of a new joint between two membraneous bones (squamosal and dentary), it was critical that an articulation surface was created between them. This problem was solved by the development of a secondary cartilage on the condylar process of the dentary to create a synovial joint at the jaw articulation. In humans the squamosal fuses with the tympanic, petrosal, styloid process and mastoid to form the compound temporal bone, and the jaw joint is given the name temporomandibular joint (TMJ). The TMJ is a sliding joint made up of the glenoid fossa of the upper jaw, and condylar of the dentary separated by a disc (). The cavity between the condylar and disc (inferior) allows for rotation of the jaw during initial mouth opening, while the cavity between the disc and glenoid fossa (superior) allows for translational (gliding) movement as the jaw opens wide. The disc develops from the condylar as a sheet of cells that lift off to create the lower synovial cavity, this process involving the hedgehog signalling pathway (Shibukawa et al. 2007; Purcell et al. 2009). The formation of a disc is therefore only possible due to the initial development of the condylar. The disc is associated with the discomallear ligament, which corresponds to a remnant of the lateral pterygoid muscle, which attaches to the caudal end of Meckel’s cartilage during embryonic development (Ogutcen-Toller, 1995; Cheynet et al. 2003). In reptiles and birds this muscle inserts on the quadrato-articular joint. The discomalleal ligament provides a connection between the malleus and jaw, along with the sphenomandibular ligament, which will be discussed later. In clinical cases, where the TMJ fails to form, the malleus and incus take over some of the role of jaw articulation, these elements therefore reverting to the role of jaw support (Herring, 1993).
The mammalian jaw articulation (A,C) MicroCT images of an adult mouse. (A) Condylar head of the dentary bone fitting into the glenoid fossa of the upper jaw. (B) Frontal section through an adult mouse jaw joint. The disc is sandwiched between the glenoid ...
Specialisation of the proximal dentary
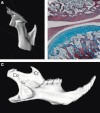
The evolution of the squamosal dentary joint has resulted in a greater level of complexity of the dentary in mammals compared with non-mammalian tetrapods. The prototypical tetrapod dentary is a simple tooth-bearing bone forming one part of the compound mandible, along with the angular, surangular, prearticular, splenial, and coronoid. In contrast, the mammalian dentary alone forms the mandible and is highly modular in nature, taking on all the functions of the membraneous bones of the lower jaw in non-mammals. The proximal dentary typically possesses three processes. These are the joint-forming condylar and two un-opposed processes which act as muscle attachment sites: the coronoid superior/rostral to the condylar, and the angular, inferior/ventral to the condylar (). These structures are not homologous to the angular and coronoid process of the non-mammalian mandible but they serve similar functional roles. For example, the coronoid process of a fish and a mammal both act as muscle attachment sites for the jaw muscles but one is an endochondrial bone derived from Meckel’s cartilage (fish) and the other is a part of the dentary, a membraneous bone.
The modular nature and evolutionary plasticity of the mammalian dentary bone has allowed for morphological variation so that mammals have been able to exploit the maximum range of dietary niches and the variation in mechanical load of different foods. The different mechanical loads acting on the dentary, arising as a consequence of different feeding strategies, result in changes to the shape and size of the angular and condylar processes evident between species, even closely related ones. For example, within Muridae, Old World rats and mice, herbivorous species require strong jaw closure muscles to generate a lateral force in the chewing action to process a diet high in tough cellulose. This has resulted in an angular process, where the relevant muscle attachment is larger than that of closely related but omnivorous species whose diet is less cellulose-rich (Michaux et al. 2007). Similarly, the giant anteater, Myrmecophaga tridactyla, does not need to generate much force during jaw closure and, consequently, the coronoid process is virtually absent and the non-articulating mandibular processes are vestigial or lost. Furthermore, as the angle of the jaw opening is only required to be minimal, the condylar process is small and the glenoid fossa shallow (Naples, 1999). In striking contrast, the large gape and strong bite of the hippopotamus has resulted in a large angular process, to ensure a large surface area for muscle attachment and a robust condylar process (Anthwal & Tucker, 2012).
Influence of secondary cartilage and mechanical force
In the mammalian dentary, one or more of the proximal processes (coronoid, angular, condylar) can be capped with a secondary cartilage, the condylar plus one or two others. These secondary cartilages, which undergo secondary ossification, act in the growth and patterning of the embryonic mandible, in addition to the role of the condylar cartilage in forming the articulation and disc.
The distribution of secondary cartilage on the proximal processes of the dentary is species-specific, with the position, size and persistence of such cartilages acting to shape the relative size of the coronoid, angular process and dentary as a whole. For example, in humans the angular process looks very similar to the mouse angular process during early development but in humans this process is not capped by secondary cartilages and fails to extend out, resulting in formation of the curved human angle. In contrast, in the mouse the angular process is capped by a robust secondary cartilage that leads to growth of this process and a pronounced angular process at birth (Anthwal & Tucker, 2012).
As the evolution of the mammalian jaw joint would not have been possible without the initiation of a secondary cartilage at the condylar process, it is important that we understand how such cartilages are initiated and the mechanisms behind their induction.
Secondary cartilages are not found in amphibians but are found during the development of birds at the 24 articulation sites of membranous bones, such as the quadratojugal/quadrate joint of the chick mandible (Buxton et al. 2003). In addition, avian secondary cartilages are found at the sites of muscle attachment, such as the point of insertion of the mandibular adductor muscles on the lower jaw in the duck (Solem et al. 2011). The pattern of secondary cartilages in the avian jaw is species-specific, reflecting differences in diet and mechanical strain on the jaw, this pattern being controlled by the neural crest (Solem et al. 2011).
Much of the literature outlining the origin of secondary cartilages has utilised the developing chick as a model. In this system, quadratojugal-quadrate joint secondary cartilages develop from Runx2-positive cells of the periosteum of the quadratojugal bone in response to mechanical stimulation and initiation of the HMG box containing transcription factor Sox9, a regulator of Type II collagen (Hall & Herring, 1990; Zhao et al. 1997; Buxton et al. 2003; Archer et al. 2006). However, the secondary cartilages of the mammal dentary processes appear to have a different developmental programme, with Sox9 acting alongside Runx2 to initiate membranous ossification from the mandibular mesenchyme (Shibata et al. 2006). In addition, mouse secondary cartilages are able to develop in the absence of mechanical stimulation, as demonstrated by the culturing of dentary explants in the absence of movement (Anthwal et al. 2008), and by the presence of mandibular secondary cartilage in knockout mice that lack muscle (Rot-Nikcevic et al. 2007). Furthermore, experiments in our lab have suggested that secondary cartilage develops as a sesamoid in explant cultures which fail to produce bone, indicating that secondary cartilage can develop regardless of membranous ossification Anthwal et al. 2008; N. Anthwal, Y. Chai, A.S. Tucker, unpublished data). Histological studies in rat also suggest that the condylar cartilage develops as a sesamoid (Vinkka, 1982). Taken together, these data suggest that in contrast to the situation in chick, the secondary cartilages of rodents do not develop from the periosteum of membranous bone, rather that secondary cartilages and membranous bone develop from the same population of skeletoblasts. In the mouse, secondary cartilages are malformed or lost in Alk2 mutants and after loss of transforming growth factor beta (Tgfβ) signalling (Dudas et al. 2004; Oka et al. 2007; Anthwal et al. 2008).
There is some debate as to whether reptiles are able to form secondary cartilages. Rieppel suggests that reptiles can do so during fracture repair (Rieppel, 1993). However, an alternative study by Irwin was unable to detect secondary cartilages in incisions made in the bone of 19 reptile specimens, 17 lizards from three species and two snakes from two species, suggesting that only birds and mammals are capable of secondary cartilage induction (Irwin & Ferguson, 1986). The uncertain status of reptilian secondary cartilages suggests the possibility that secondary cartilages have evolved independently. This hypothesis is certainly supported by the differences in avian and mammalian secondary cartilage function and development. The term ‘secondary cartilage’ is a very broad one, including as it does mechanically induced cartilages, cartilages that develop due to fracture of membranous bones and cartilages that develop as part of the normal patterning of a membranous bone (Beresford, 1981). It may be useful to distinguish architectural secondary cartilages, which have a role in the normal morphogenesis of a membranous bone such as the condylar cartilage, and reactive secondary cartilages, which develop in reaction to an external force. In keeping with a need to define more accurately secondary cartilages, it has recently been shown that in avian embryos, secondary cartilage that forms at articulation sites is controlled by different mechanisms than closely developing secondary cartilage that forms at the insertion of muscles (enthesis) (Solem et al. 2011). Enthesis and articular cartilage both require mechanical force to form, but express a different pattern of genes and respond differently to block of ion channels. Thus secondary cartilages, although morphologically similar, are formed by very different mechanisms within and between species, making accurate comparisons difficult.
Tissue interactions between the dentary and upper jaw
The evolutionary elaboration of the dentary to form a condylar process has also required the addition of an articulation site within the squamosal bone. This articulation site takes the form of the glenoid fossa, a cavity within the squamosal upon which the condylar head sits to form the hinge of the jaw. Much like the specialisation of the dentary, the evolution of the glenoid fossa has required the utilisation of a membranous bone in a mobile joint. However, unlike the condylar process, no secondary cartilages have formed. As with the rest of the jaw articulation, little is known about the development of the glenoid fossa, although one recent study has revealed that interaction with the condylar is vital for its formation. Conditional knockout mouse strategies were used to disrupt the development of the condylar process: genetic ablation of the condylar cartilage with Wnt1Cre;Sox9flox/flox mutants, and dislocation of the joint with K14Cre;catnbf(ex3) mutants (Wang et al. 2011). In both cases the glenoid fossa was initiated but later regressed, indicating that tissue interaction between the condylar cartilage and squamosal are required for the development of a glenoid fossa. The use of mice with a mutant allele of the BMP antagonist Noggin, which experience a rapid expansion of Meckel’s cartilage thus displacing the condylar from the articulation with the cranial base, enabled the sustained development of a glenoid fossa, albeit with an non-functional joint (Wang et al. 2011). This study suggests that the squamosal is competent to form a glenoid cavity in the presence of an articulating element, which need not be the condylar portion of the dentary. Intriguingly, the fossil record offers one example of such a situation: Probainognathus, a late Jurassic cynodont, possessed a jaw articulation formed between the glenoid fossa of the squamosal and the surangular, an intramembraneous element of the non-mammalian tetrapod mandible lost in modern mammals (reviewed Luo, 2011). In addition to signals from the condylar to the fossa, there also appear to be signals from the fossa to the condylar, resulting in generation of a disc, which fails to form if the condylar does not contact the fossa (Wang et al. 2011). Signalling is therefore in both directions to allow coordinated development of the upper and lower jaws.
Fate of Meckel’s cartilage in mammals
In birds and reptiles Meckel’s cartilage remains unossified, with the exception of the retroarticular process, and persists as a core providing flexible structural support for the lower jaw bones that wrap around it (). In mammals such support in the adult is perhaps unnecessary, as the single dentary forms the lower jaw, and as a consequence much of Meckel’s cartilage does not persist into adulthood.
The fate of the mammalian Meckel’s is complex and differs along its length, reflecting its different environment and function. The most proximal (caudal) part, as has been discussed, undergoes endochondrial ossification, and forms the malleus and incus (Amin & Tucker, 2006). This region of Meckel’s has been shown to be Type X collagen positive, a marker for transformation to bone (Chung & Nishimura, 1999). The most distal (rostral) tips form the rostral process that joins the two arms of Meckel’s cartilage at the symphysis (Bhaskar et al. 1953). In humans, this part of Meckel’s cartilage persists, forming small nodules on the dorsal surface of the symphysis (Rodriguez-Vazquez et al. 1997). In the rat, the hyaline cartilage at the rostal tip is replaced by fibrocartilage at the time of weaning, as the animal moves from sucking to mastication (Bhaskar et al. 1953). The rostral cartilage is lost when Alk2 is conditionally knocked out in the neural crest (using Wnt1 cre). This results in loss of the symphysis and the mandible bones remain separate (Dudas et al. 2004). Alk2 is a type I receptor for BMP signalling, indicating the important role the Bmps play in the development of the rostral part of Meckel’s cartilage.
Meckel’s cartilage in the mid portion (running from the molar primordium to the rostral process) can directly undergo ossification or be resorbed to be replaced by bone (Richman & Diewert, 1988; Rodriguez-Vazquez et al. 1997; Harada & Ishizeki, 1998). In explant culture, cells of Meckel’s cartilage have been shown to be able to develop into osteoblasts (Richman & Diewert, 1988). Resorption commences with a breakdown of the perichondrium, followed by invasion of vasculature and an infiltrate of TRAP-positive osteoclasts/chondroclasts, and macrophages that engulf the chondrocytes (Harada & Ishizeki, 1998). Chondrocytes of Meckel’s cartilage may regulate their own cell fate through matrix remodelling and expression of matrix-metallo-proteinases (MMPs) (Sakakura et al. 2007). MMPs are a family of proteases that degrade protein components of the extracellular matrix. In this way they may influence cellular development by altering the composition of ECM components such as growth factors and cytokines. MMPs are highly expressed in rheumatoid arthritis, a pathological example of the resorption of uncalcified matrix. A range of MMPs are associated with the mid-rostral section of Meckel’s cartilage and may contribute to resorption. Interestingly, the specific pattern of MMPs appears unique to Meckel’s cartilage and is different from those found in limb cartilages undergoing endochondrial ossification (Sakakura et al. 2007).
Despite this region of Meckel’s not functioning as an adult structure, it plays an integral supporting role as a scaffold for the mandible throughout embryonic development with defects in Meckel’s causing malformations of the developing mandible (Ito et al. 2002; Dudas et al. 2004). In humans, Meckel’s cartilage acts as an initial attachment site for the muscles of the mandible. Once these muscles lose contact with Meckel’s and reach the developing dentary, transformation of Meckel’s cartilage commences (Wyganowska-Swiatkowska & Przystanska, 2011). Loss of muscle attachment to Meckel’s may therefore be a possible stimulus for a change of fate for Meckel’s cartilage.
Breaking the connection between the ear and jaw
A key step in the formation of the definitive mammalian middle ear (DMME) was the breakdown of Meckel’s cartilage between the forming dentary and malleus. From the fossil record it is clear that the articular and quadrate were already playing a part in hearing and had lined up with the stapes in the middle ear before they became physically separated from the jaw. Breakdown of Meckel’s cartilage is thus the final step in the move towards the definitive mammalian ear. Although the mechanisms of removal are unclear, the consequence is to free the first two ossicles of the middle ear from the rest of the cartilage, in this way removing the connection between the jaw and middle ear and functionally separating the feeding and hearing apparatuses. This is an adaptation seen in all living adult mammals today.
The timing of the break varies between species. As has already been mentioned, this occurs relatively late in marsupials, at around P20, but a late break is also observed in the placental tree shrew (Tupaia), where Meckel’s remains connecting the ear to the jaw until around P14, and is thought to function as a skeletal support (Zeller, 1987). In humans, Meckel’s cartilage breaks down by the 8th month of gestation (Cheynet et al. 2003). In clinical cases where the temporomandibular joint does not develop, Meckel’s remains continuous (Herring, 1993). The stimulus for breakdown of Meckel’s may therefore be linked to development of a functional TMJ.
During development, the stretch of Meckel’s cartilage from the molar primordium to the malleus undergoes an unusual transformation to form a fibrous ligamentous structure (Harada & Ishizeki, 1998), which develops into the sphenomandibular and anterior malleolar ligaments (Ogutcen-Toller, 1995; Cheynet et al. 2003). The sphenomandibular (also known as the malleomandibular) is involved in TMJ movement by limiting the distension of the mandible and preventing dislocation and forms between the sphenoid bone and dentary. The connected anterior malleolar ligament attaches to the malleus and anterior wall of the tympanic cavity and acts to stabilise the mandible.
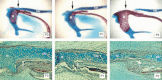
The transformation process has been studied in the mouse and rat. By postnatal day 3 in mice this portion of Meckel’s cartilage no longer stains with Alcian Blue, a dye for cartilage-associated matrix proteoglycans (). In histological section the cartilage can be seen to thin in this region, the rounded cartilage cells disappearing, to be replaced by elongated fibroblastic cells (). The transformation process starts close to the malleus and then spreads out in a wave towards the dentary. No invasion of vasculature, macrophages or osteoclasts was observed in this part of Meckel’s (Harada & Ishizeki, 1998). Organ culture of this region of Meckel’s cartilage in the rat shows that isolated cartilage that has been stripped of the periochondrium differentiates into cells of fibroblast morphology, which do not express cartilage specific proteoglycans typical of chondrocytes (Richman & Diewert, 1988). It is the cartilage cells themselves, therefore, rather than the perichondrium, that undergo this transformation. Cells in this region do not proliferate to give rise to fibroblasts, suggesting the potential to alter their cell fate from chondrocytes and transform directly into fibroblasts themselves. Cell death is not observed within this mid region although it is seen in the perichondrium of the mandibular and auricular regions (Trichilis & Wroblewski, 1997; Harada & Ishizeki, 1998).
Breakdown of Meckel’s cartilage (A–C) Skeletal preps of the mouse malleus and Meckel’s cartilage. Red – bone stained by Alizarin Red. Blue – cartilage stained by Alcian Blue. (A) P0. (B) P1. (C) P3. (D–F) ...
Meckel’s cartilage has been successfully cultured in vitro and has proved a valuable tool for assessing the competence of Meckel’s to undergo different fates and look at the role of growth factors (Richman & Diewert, 1988; Harada & Ishizeki, 1998; Ishizeki et al. 2001). When Meckel’s cartilage from rat embryos at embryonic day (E)17 was cultured in ocular grafts, the different regions of Meckel’s continued to develop into their normal fates (bone, ligament, etc.) (Richman & Diewert, 1988). This suggests rat chondrocytes of Meckel’s cartilage from E17 onwards are specified to follow their terminal cell fates. However, when mouse E17 Meckel’s cartilage was dissected and cultured in dishes it was found to ossify (Ishizeki et al. 2001). The default state for this region of Meckel’s therefore appears to be to undergo ossification. A similar result was found when Meckel’s chondrocytes from mouse E17 embryos were grown in tissue culture, the cells expressing Type X collagen and osteocalcin and forming nodules with calcification of the matrix, indicating a transformation to osteogenic cells (Ishizeki et al. 1997). Clinical cases where Meckel’s fails to breakdown and ossifies have been reported in human fetuses, but these are very rare (Keith, 1910).
That Meckel’s cartilage forms bone when isolated during development fits well with the finding of an ossified Meckel’s in the fossil record. If the default state of Meckel’s cartilage is to ossify, then an important step in mammalian evolution would have been the prevention of ossification in this region. Studies have implicated two signalling pathways in this step, epidermal growth factor (EGF) and transforming growth factor beta (TGFβ).
For example, ossification of Meckel’s cartilage, as indicated by staining for Alizarin Red, could be prevented in Meckel’s cartilage cultures by the addition of epidermal growth factor (EGF). Instead of formation of a mineralized matrix, the cells transformed to a ligamentous fate, as they would have done in the embryo (Ishizeki et al. 2001). Addition of EGF caused cells to proliferate, downregulate cartilage type proteoglycans and adopt fibroblastic morphologies that expressed type 1 collagen. Interestingly these alterations in response to EGF were uniform throughout Meckel’s cartilage, suggesting that chondrocytes of Meckel’s cartilage have homogeneous potential to respond to EGF (Ishizeki et al. 2001).
To investigate the effect of EGF in vivo, EGF was injected into newborn mice. This resulted in an accelerated disappearance of Meckel’s cartilage, indicated by a more rapid loss of Alcian Blue staining between the dentary and the ossicles.
Taken together, this study implicates EGF as potential inducer of chondrocyte to fibroblast transformation, suggesting EGF may control the boundaries and formation of the sphenomandibular ligament in vivo.
In the TGFbr2 knockout, in addition to a number of defects associated with the dentary, Meckel’s cartilage ossifies at birth (Oka et al. 2007; Anthwal et al. 2008). This ossification occurs in the mid region, which would usually be resorbed and transformed into the sphenomandibular ligament. In Tgfbr2 mutants, Dlx 5 expression is upregulated (Oka et al. 2008) and, intriguingly, an ossified Meckel’s is also observed in Dlx5−/− mutants and Dlx1−/−; Dlx 5+/1 double mutants (Depew et al. 2005). Lack of Tgfβ signalling causes Indian hedgehog (Ihh), a gene expressed in differentiating but not immature chondrocytes, to have an expanded expression domain, implying that loss of Tgfβ causes accelerated differentiation of chondrocytes (Oka et al. 2007). In normal mammalian development, the mid region of Meckel’s would receive a signal to transform to a ligamentous fate. If this signal is not received, as in the case of the cultures of Meckel’s cartilage, these cells will follow a bone differentiation pathway, or if these cells start on an ossification pathway before they receive the signal, as in the case of Tgfbr2 mutants, then an ossified connection is created between the mandible and middle ear. Therefore Tgfβ signalling may indirectly control the fate of the mid region by slowing down chondrocyte differentiation to maintain responsiveness to signalling factors that initiate the transformation of this region. Most likely, Tgfβ signalling will act in concert with other growth factors such as EGF to control developmental timing of proliferation, differentiation and therefore the fate of chondrocytes in Meckel’s cartilage. The temporary presence of Meckel’s cartilage during embryonic and early postnatal stages in living mammals may implicate the ossified Meckel’s cartilage (OMC) in ancestral mammals, an example of paedomorphosis, the retention of an embryonic structure in the mature adult (Ji et al. 2009; Luo, 2011). Paedomorphosis is a result of developmental heterochrony, that is, the shift in timing of a particular genetic pathway. Therefore the timing of Tgfβ signalling, EGF signalling, chondrocyte differentiation and ossification could perhaps have been a determining factor for the fate of the mid portion of Meckel’s cartilage and in this way determined whether it was retained as an ossified adult structure or transformed into a ligament.
Conclusion
In conclusion, the evolution of the mammalian middle ear and jaw joint were pivotal steps in the evolution of mammals. It is also a great example of how classical comparative anatomy, paleontology and developmental biology have come together to piece together how this remarkable transformation of jaw joint to ear ossicles was able to come about. The homologies of the malleus, incus and stapes to the articular, quadrate and columella, and tympanic ring and gonial to the angular and prearticular suggested by comparative anatomy 175 years ago have been recently confirmed by molecular and developmental biology. The recent discovery of new mammaliform fossils has allowed careful documentation of the shift from primary to secondary jaw articulation, creating an opportunity to follow the transformation of the post-dentary skeletal elements. This fossil data has been complemented by the study of marsupial development, providing insight into the changing role of the malleus and incus, and the relationship of the primary and secondary jaw joints. We now have a number of unanswered questions. What are the signalling molecules involved in the interactions between the condylar process and the glenoid fossa that create the novel mammalian jaw joint? What are the mechanisms that lead to transformation of Meckel’s cartilage into a ligament allowing isolation of the ear from the jaw? What controls the distribution of secondary cartilages? What controls the timing of differentiation and cessation of growth of the ossicles relative to the jaw? With the new tools available to us we hope to be able to address some of these questions and provide insights into the mechanisms that lie behind evolution.
Acknowledgments
Thanks to Robert Asher and Zhe-Xi Luo for discussions on the evolution of the ear. Thanks to Kathleen Smith for the image of the Monodelphis neonate. Thanks to Hannah Thompson for her comparative diagram of the middle ear (). Leena Joshi is supported by Deafness Research UK. Abigail Tucker is supported by the Medical Research Council (MRC). The authors declare no conflict of interest.
References
- Allin EF, Hopson JA. Evolution of the auditory system in Synapsida (‘mammal-like reptiles’ and primitive mammals) as seen in the fossil record. In: Webster DB, Fay RR, Popper AN, editors. The Evolutionary Biology of Hearing. New York: Springer-Verlag; 1992. pp. 587–614.
- Amin S, Tucker AS. Joint formation in the middle ear: lessons from the mouse and guinea pig. Dev Dyn. 2006;235:1326–1333. [PubMed]
- Anthwal N, Tucker AS. Molecular biology of the mammalian dentary: insights into how complex skeletal elements can be shaped during development and evolution. In: Asher RJ, Muller J, editors. Clone to Bone: The Synergy of Morphological and Molecular Tools in Palaeobiology. Cambridge: Cambridge University Press; 2012. pp. 396–426. Chapter 8.
- Anthwal N, Chai Y, Tucker AS. The role of transforming growth factor-beta signalling in the patterning of the proximal processes of the murine dentary. Dev Dyn. 2008;237:1604–1613. [PubMed]
- Archer CW, Buxton P, Hall BK, et al. Mechanical regulation of secondary chondrogenesis. Biorheology. 2006;43:355–370. [PubMed]
- Benton MJ. Vertebrate Palaeontology. New York: Wiley-Blackwell; 2005.
- Beresford WA. Chondroid Bone, Secondary Cartilage and Metaplasia. Baltimore: Urban & Schwarzenberg; 1981.
- Bhaskar SN, Weinmann JP, Schour I. Role of Meckel’s cartilage in the development and growth of the rat mandible. J Dent Res. 1953;32:398–410. [PubMed]
- Buxton PG, Hall B, Archer CW, et al. Secondary chondrocyte-derived Ihh stimulates proliferation of periosteal cells during chick development. Development. 2003;130:4729–4739. [PubMed]
- Cheynet F, Guyot L, Richard O, et al. Discomallear and malleomandibular ligaments: anatomical study and clinical applications. Surg Radiol Anat. 2003;25:152–157. [PubMed]
- Chung KS, Nishimura I. Maintenance of regional histodifferentiation patterns and a spatially restricted expression of type X collagen in rat Meckel’s cartilage explants in vitro. Arch Oral Biol. 1999;44:489–497. [PubMed]
- Clark CT, Smith KK. Cranial osteogenesis in Monodelphis domestica (Didelphidae) and Macropus eugenii (Macropodidae) J Morphol. 1993;215:119–149. [PubMed]
- Clouthier DE, Hosoda K, Richardson JA, et al. Cranial and cardiac neural crest defects in endothelin-A receptor-deficient mice. Development. 1998;125:813–824. [PubMed]
- Couly GF, Coltey PM, Le Douarin NM. The triple origin of skull in higher vertebrates: a study in quail-chick chimeras. Development. 1993;117:409–429. [PubMed]
- Crompton AW. On the lower jaw of Diartrognathus and the origin of the mammalian lower jaw. Proc Zool Soc London. 1963;140:697–753.
- Crompton AW, Hylander WL. Changes in mandibular function following the acquisition of a dentary-squamosal joint. In: Hotton N, MacLean PD, Roth JJ, Roth EC, editors. The Ecology and Biology of Mammal-like Reptiles. Washington, DC: Smithsonian Institute Press; 1986. pp. 78–98.
- Depew MJ, Lufkin T, Rubenstein JL. Specification of jaw subdivisions by Dlx genes. Science. 2002;298:381–385. [PubMed]
- Depew MJ, Simpson CA, Morasso M, et al. Reassessing the Dlx code: the genetic regulation of branchial arch skeletal pattern and development. J Anat. 2005;207:501–561. [PMC free article] [PubMed]
- Derobert Y, Plouhinec JL, Sauka-Spengler T, et al. Structure and expression of three Emx genes in the dogfish Scyliorhinus canicula: functional and evolutionary implications. Dev Biol. 2002;247:390–404. [PubMed]
- Dudas M, Sridurongrit S, Nagy A, et al. Craniofacial defects in mice lacking BMP type I receptor Alk2 in neural crest cells. Mech Dev. 2004;121:173–182. [PubMed]
- Filan SL. Development of the middle ear region in Monodelphis domestica (Marsupial, Didelphidae): marsupial solutions to an early birth. J Zool Lond. 1991;225:577–588.
- Galli A, Roure A, Zeller R, et al. Glypican 4 modulates FGF signalling and regulates dorsoventral forebrain patterning in Xenopus embryos. Development. 2003;130:4919–4929. [PubMed]
- Gaupp E. De Reichertsche Theorie. (Hammer-, amboss- und Kerferfrage) Arch Anat Physiol. 1912;(Suppl):1–416.
- Gendron-Maguire M, Mallo M, Zhang M, et al. Hoxa-2 mutant mice exhibit homeotic transformation of skeletal elements derived from cranial neural crest. Cell. 1993;75:1317–1331. [PubMed]
- Hall BK. Bones and cartilage: Developmental and Evolutionary Skeletal Biology. San Diego: Elsevier; 2005.
- Hall BK, Herring SW. Paralysis and growth of the musculoskeletal system in the embryonic chick. J Morphol. 1990;206:45–56. [PubMed]
- Harada Y, Ishizeki K. Evidence for transformation of chondrocytes and site-specific resorption during the degradation of Meckel’s cartilage. Anat Embryol (Berl) 1998;197:439–450. [PubMed]
- Herring SW. Formation of the vertebrate face: epigenetic and functional influences. Am Zool. 1993;33:472–483.
- Irwin CR, Ferguson MW. Fracture repair of reptilian dermal bones: can reptiles form secondary cartilage? J Anat. 1986;146:53–64. [PMC free article] [PubMed]
- Ishizeki K, Hiraki Y, Kubo M, et al. Sequential synthesis of cartilage and bone marker proteins during transdifferentiation of mouse Meckel’s cartilage chondrocytes in vitro. Int J Dev Biol. 1997;41:83–89. [PubMed]
- Ishizeki K, Takahashi N, Nawa T. Formation of the sphenomandibular ligament by Meckel’s cartilage in the mouse: possible involvement of epidermal growth factor as revealed by studies in vivo and in vitro. Cell Tissue Res. 2001;304:67–80. [PubMed]
- Ito Y, Bringas P, Jr, Mogharei A, et al. Receptor-regulated and inhibitory Smads are critical in regulating transforming growth factor beta-mediated Meckel’s cartilage development. Dev Dyn. 2002;224:69–78. [PubMed]
- Ji Q, Luo ZX, Zhang X, et al. Evolutionary development of the middle ear in Mesozoic therian mammals. Science. 2009;326:278–281. [PubMed]
- Keith A. Abnormal ossification of Meckel’s cartilage. J Anat Physiol. 1910;44:151–152. [PMC free article] [PubMed]
- Kemp TS. The Origin and Evolution of Mammals. Oxford: Oxford University Press; 2005.
- Kermack KA. The origin of mammals and the evolution of the temporomandibular joint. Proc R Soc Med. 1972;65:389–392. [PMC free article] [PubMed]
- Kermack KA, Musset F. The ear in mammal-like reptiles and early mammals. Acta Palaeontol Pol. 1983;28:147–158.
- Kermack KA, Mussett AF, Rigney HW. The skull of Morganucodon. Zool J Linn Soc. 1981;71:1–158.
- Kielan-Jaworowska Z, Cifelli RL, Luo Z-X. Mammals from the Age of Dinosaurs: Origins, Evolution, and Structure. New York: Columbia University Press; 2004.
- Kontges G, Lumsden A. Rhombencephalic neural crest segmentation is preserved throughout craniofacial ontogeny. Development. 1996;122:3229–3242. [PubMed]
- Luo Z-X. Developmental patterns in Mesozoic evolution of mammal ears. Annu Rev Ecol Evol Syst. 2011;42:355–380.
- Luo ZX, Crompton AW, Sun AL. A new mammaliaform from the early Jurassic and evolution of mammalian characteristics. Science. 2001;292:1535–1540. [PubMed]
- Luo ZX, Chen P, Li G, et al. A new eutriconodont mammal and evolutionary development in early mammals. Nature. 2007;446:288–293. [PubMed]
- Maier W. Der Processus angularis bei Monodelphis domestica und seine Beziehungen zum Mittelohr. Eine ontogenetische und evolutionsmorphologische Untersuchung. Gegenbaurs Morphol Jahrb Leipzig. 1987;133:123–161. [PubMed]
- Maier W. Phylogeny and ontogeny of mammalian middle ear structures. Neth J Zool. 1990;40:55–74.
- Meng J, Wang Y, Li C. Transitional mammalian middle ear from a new Cretaceous Jehol eutriconodont. Nature. 2011;472:181–185. [PubMed]
- Michaux J, Chevret P, Renaud S. Morphological diversity of Old World rats and mice (Rodentia, Muridae) mandible in relation with phylogeny and adaptation. J Zoolog Syst Evol Res. 2007;45:263–279.
- Miller CT, Yelon D, Stainier DY, et al. Two endothelin 1 effectors, hand2 and bapx1, pattern ventral pharyngeal cartilage and the jaw joint. Development. 2003;130:1353–1365. [PubMed]
- Muller F. [Transitory closures in the postnatal development of Marsupialia] Acta Anat (Basel) 1968;71:581–624. [PubMed]
- Naples VL. Morphology, evolution and function of feeding in the giant anteater (Myrmecophaga tridactyla) J Zool. 1999;249:19–41.
- O’Gorman S. Second branchial arch lineages of the middle ear of wild-type and Hoxa2 mutant mice. Dev Dyn. 2005;234:124–131. [PubMed]
- Ogutcen-Toller M. The morphogenesis of the human discomalleolar and sphenomandibular ligaments. J Craniomaxillofac Surg. 1995;23:42–46. [PubMed]
- Oka K, Oka S, Sasaki T, et al. The role of TGF-beta signaling in regulating chondrogenesis and osteogenesis during mandibular development. Dev Biol. 2007;303:391–404. [PMC free article] [PubMed]
- Oka K, Oka S, Hosokawa R, et al. TGF-beta mediated Dlx5 signaling plays a crucial role in osteo-chondroprogenitor cell lineage determination during mandible development. Dev Biol. 2008;321:303–309. [PMC free article] [PubMed]
- Ozeki H, Kurihara Y, Tonami K, et al. Endothelin-1 regulates the dorsoventral branchial arch patterning in mice. Mech Dev. 2004;121:387–395. [PubMed]
- Presley R, Steel FL. The pterygoid and ectopterygoid in mammals. Anat Embryol. 1978;154:95–110. [PubMed]
- Purcell P, Joo BW, Hu JK, et al. Temporomandibular joint formation requires two distinct hedgehog-dependent steps. Proc Natl Acad Sci U S A. 2009;106:18297–18302. [PMC free article] [PubMed]
- Qiu M, Bulfone A, Martinez S, et al. Null mutation of Dlx-2 results in abnormal morphogenesis of proximal first and second branchial arch derivatives and abnormal differentiation in the forebrain. Genes Dev. 1995;9:2523–2538. [PubMed]
- Reichert KB. Über die Visceralbogen der Wirbelthiere im Allgemeinen und deren Metamorphosen bei den Vögeln und Säugethieren. Arch Anat Physiol Wissensch Med. 1937;1837:120–220.
- Rhodes CR, Parkinson N, Tsai H, et al. The homeobox gene Emx2 underlies middle ear and inner ear defects in the deaf mouse mutant pardon. J Neurocytol. 2003;32:1143–1154. [PubMed]
- Richman JM, Diewert VM. The fate of Meckel’s cartilage chondrocytes in ocular culture. Dev Biol. 1988;129:48–60. [PubMed]
- Rieppel O. Patterns of diversity in the reptilian skull. In: Hanken J, Hall BK, editors. The Skull. Chicago: The University of Chicago Press; 1993. pp. 344–390.
- Rijli FM, Mark M, Lakkaraju S, et al. A homeotic transformation is generated in the rostral branchial region of the head by disruption of Hoxa-2, which acts as a selector gene. Cell. 1993;75:1333–1349. [PubMed]
- Rodriguez-Vazquez JF, Merida-Velasco JR, Merida-Velasco JA, et al. Development of Meckel’s cartilage in the symphyseal region in man. Anat Rec. 1997;249:249–254. [PubMed]
- Rot-Nikcevic I, Downing KJ, Hall BK, et al. Development of the mouse mandibles and clavicles in the absence of skeletal myogenesis. Histol Histopathol. 2007;22:51–60. [PubMed]
- Rowe T. Coevolution of the mammalian middle ear and neocortex. Science. 1996;273:651–654. [PubMed]
- Ruest LB, Xiang X, Lim KC, et al. Endothelin-A receptor-dependent and -independent signaling pathways in establishing mandibular identity. Development. 2004;131:4413–4423. [PMC free article] [PubMed]
- Sakakura Y, Hosokawa Y, Tsuruga E, et al. Contributions of matrix metalloproteinases toward Meckel’s cartilage resorption in mice: immunohistochemical studies, including comparisons with developing endochondral bones. Cell Tissue Res. 2007;328:137–151. [PubMed]
- Sanchez-Villagra MR, Gemballa S, Nummela S, et al. Ontogenetic and phylogenetic transformations of the ear ossicles in marsupial mammals. J Morphol. 2002;251:219–238. [PubMed]
- Sato T, Kurihara Y, Asai R, et al. An endothelin-1 switch specifies maxillomandibular identity. Proc Natl Acad Sci U S A. 2008;105:18806–18811. [PMC free article] [PubMed]
- Satokata I, Maas R. Msx1 deficient mice exhibit cleft palate and abnormalities of craniofacial and tooth development. Nat Genet. 1994;6:348–356. [PubMed]
- Shibata S, Suda N, Suzuki S, et al. An in situ hybridization study of Runx2, Osterix, and Sox9 at the onset of condylar cartilage formation in fetal mouse mandible. J Anat. 2006;208:169–177. [PMC free article] [PubMed]
- Shibukawa Y, Young B, Wu C, et al. Temporomandibular joint formation and condyle growth require Indian hedgehog signaling. Dev Dyn. 2007;236:426–434. [PubMed]
- Smith KK. Development of craniofacial musculature in Monodelphis domestica (Marsupialia, Didelphidae) J Morphol. 1994;222:149–173. [PubMed]
- Smith KK. Craniofacial development in marsupial mammals: developmental origins of evolutionary change. Dev Dyn. 2006;235:1181–1193. [PubMed]
- Smith KK, Schneider RA. Have gene knockouts caused evolutionary reversals in the mammalian first arch? BioEssays. 1998;20:245–255. [PubMed]
- Solem RC, Eames BF, Tokita M, et al. Mesenchymal and mechanical mechanisms of secondary cartilage induction. Dev Biol. 2011;356:28–39. [PMC free article] [PubMed]
- Takechi M, Kuratani S. History of studies on mammalian middle ear evolution: a comparative morphological and developmental biology perspective. J Exp Zool B Mol Dev Evol. 2010;314:417–433. [PubMed]
- Trichilis A, Wroblewski J. Expression of p53 and hsp70 in relation to apoptosis during Meckel’s cartilage development in the mouse. Anat Embryol (Berl) 1997;196:107–113. [PubMed]
- Tucker AS, Watson RP, Lettice LA, et al. Bapx1 regulates patterning in the middle ear: altered regulatory role in the transition from the proximal jaw during vertebrate evolution. Development. 2004;131:1235–1245. [PubMed]
- Vinkka H. Secondary cartilages in the facial skeleton of the rat. Proc Finn Dent Soc. 1982;78(Suppl 7):1–137. [PubMed]
- Wang Y, Hu Y, Meng J, et al. An ossified Meckel’s cartilage in two Cretaceous mammals and origin of the mammalian middle ear. Science. 2001;294:357–361. [PubMed]
- Wang Y, Liu C, Rohr J, et al. Tissue interaction is required for glenoid fossa development during temporomandibular joint formation. Dev Dyn. 2011;240:2466–2473. [PMC free article] [PubMed]
- Wilson J, Tucker AS. Fgf and Bmp signals repress the expression of Bapx1 in the mandibular mesenchyme and control the position of the developing jaw joint. Dev Biol. 2004;266:138–150. [PubMed]
- Wyganowska-Swiatkowska M, Przystanska A. The Meckel’s cartilage in human embryonic and early fetal periods. Anat Sci Int. 2011;86:98–107. [PubMed]
- Zeller U. Morphogenesis of the mammalian skull with special reference to Tupaia. In: Kuhn H-J, Zeller U, editors. Morphogenesis of the Mammalian Skull. Hamburg: Verlag Paul Parey; 1987. pp. 54–98.
- Zhao Q, Eberspaecher H, Lefebvre V, et al. Parallel expression of Sox9 and Col2a1 in cells undergoing chondrogenesis. Dev Dyn. 1997;209:377–386. [PubMed]